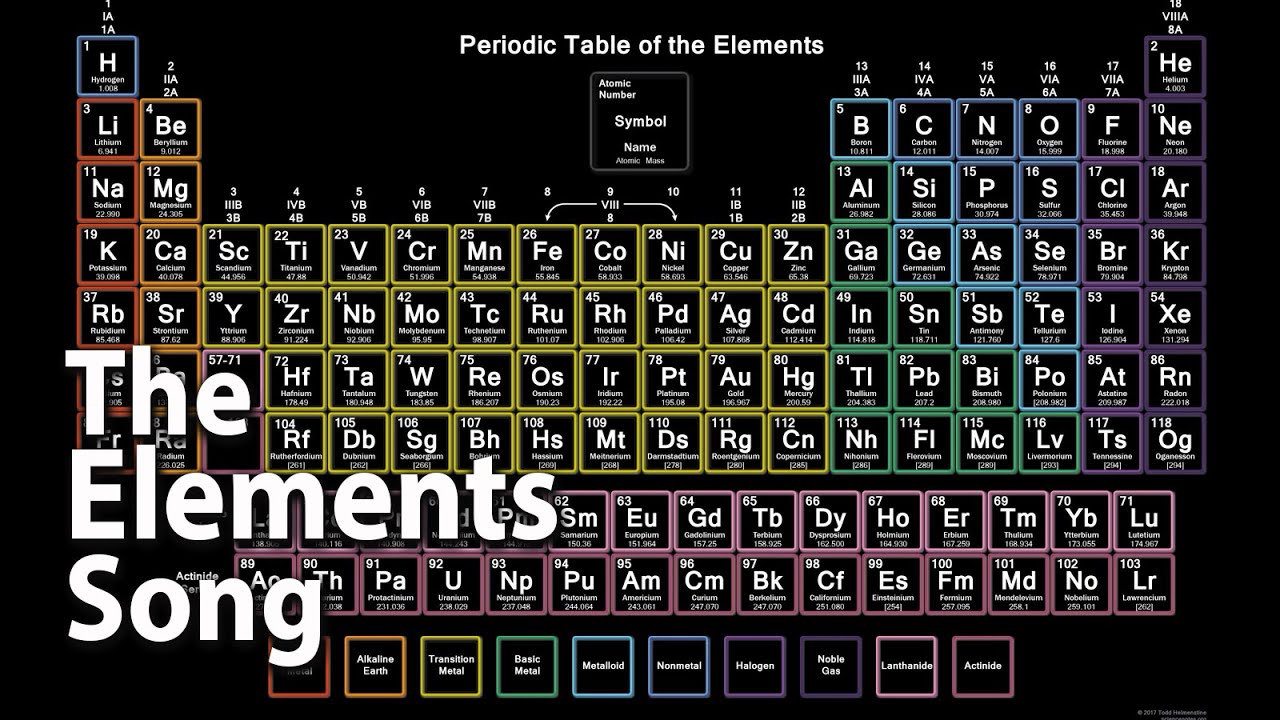
The periodic table is not just a chart of chemical elements; it is a symphony of the universe's building blocks. Every element resonates with its unique characteristics, contributing to a harmonious ensemble that forms the foundation of everything around us. The "song of elements in the periodic table" captures the essence of this scientific masterpiece, transforming it into an educational tool that is both engaging and enlightening. This intriguing concept bridges the gap between music and science, making the study of elements more accessible and enjoyable for learners of all ages.
In this article, we delve into the world of the periodic table through the lens of its musical rendition. The "song of elements in the periodic table" is a creative approach that uses rhythm and melody to teach the properties and order of elements. By exploring this concept, we aim to provide a deeper understanding of how the periodic table is organized, the significance of each element, and how they relate to one another. This innovative method not only aids in memorization but also encourages a holistic appreciation of the natural world.
Join us as we embark on a journey through the periodic table, uncovering the stories behind each element and their role in the grand symphony of chemistry. From the lightest hydrogen to the heaviest synthetic elements, we will explore the characteristics, applications, and historical significance of these fundamental components. This article serves as a comprehensive guide for students, educators, and anyone interested in the intriguing interplay between science and music.
Table of Contents
- History of the Periodic Table
- Understanding the Periodic Table
- The Significance of the 'Song of Elements'
- Elements and Their Properties
- Musical Interpretation of Elements
- Educational Benefits of the Song
- Notable Elements in the Song
- Creative Approaches to Learning Elements
- Impact on Science Education
- Future of Music and Science Integration
- Challenges in Creating the Song
- Success Stories of Using the Song
- Criticism and Limitations
- How to Use the Song in Classroom
- FAQs
- Conclusion
History of the Periodic Table
The periodic table's history is a tale of discovery and innovation. It began in the early 19th century when scientists sought to organize the known elements based on their properties. Dmitri Mendeleev, a Russian chemist, is often credited with creating the first widely recognized version of the periodic table in 1869. He arranged the elements in order of increasing atomic mass, leaving gaps for elements yet to be discovered, predicting their properties with remarkable accuracy.
Before Mendeleev, other scientists like Johann Döbereiner and John Newlands made significant contributions. Döbereiner's triads grouped elements with similar properties, while Newlands proposed the Law of Octaves, suggesting that elements repeated their properties every eighth element. These early efforts laid the groundwork for Mendeleev's breakthrough.
As new elements were discovered, the periodic table evolved. The discovery of protons and neutrons led to the modern understanding of atomic numbers, refining the classification system. Henry Moseley, in the early 20th century, reordered the table based on atomic number rather than atomic mass, resolving previous inconsistencies.
The periodic table continued to expand with the discovery of noble gases and synthetic elements. Glenn T. Seaborg's work on transuranium elements and the actinide series further shaped the table's structure. Today, the periodic table is a dynamic tool, constantly updated with new discoveries and insights into atomic theory.
Understanding the Periodic Table
The periodic table is more than a collection of elements; it is a systematic representation of chemical properties and relationships. Each element is positioned according to its atomic number, electron configurations, and recurring chemical properties. The table's structure reveals the periodicity of elements, where similar properties recur at regular intervals.
The table is divided into periods (rows) and groups (columns). Periods represent elements with the same number of electron shells, while groups contain elements with similar valence electron configurations. This arrangement explains why elements in the same group exhibit similar chemical behavior.
Metals, nonmetals, and metalloids are distinguished by their placement on the table. Metals, found on the left, are typically conductive and malleable. Nonmetals, on the right, lack these properties and include gases like oxygen and nitrogen. Metalloids, positioned between metals and nonmetals, exhibit characteristics of both.
The periodic table also includes blocks—s, p, d, and f—based on electron orbital types. Transition metals, located in the d-block, are known for their complex electron configurations, while the f-block contains the lanthanide and actinide series, often depicted separately due to their unique properties.
The Significance of the 'Song of Elements'
The "song of elements in the periodic table" offers a novel way to engage with chemistry. By setting the elements to music, this concept transforms learning into an auditory experience, appealing to diverse learning styles. The song helps students memorize elements' names, symbols, and positions, making chemistry more accessible and enjoyable.
Music has a profound impact on memory and cognition. The rhythm and melody of a song can enhance retention, allowing learners to recall information more easily. By associating elements with a catchy tune, the "song of elements in the periodic table" leverages this cognitive advantage to facilitate learning.
Furthermore, the song fosters a deeper appreciation for the periodic table's order and logic. It highlights the relationships between elements, encouraging learners to explore beyond rote memorization. This approach aligns with modern educational practices that emphasize understanding over memorization.
The "song of elements in the periodic table" also bridges the gap between science and the arts, demonstrating the interdisciplinary nature of knowledge. It encourages creativity and innovation, showcasing how different fields can complement and enhance each other.
Elements and Their Properties
Each element in the periodic table has its own unique set of properties, which define its behavior in chemical reactions and its role in the natural world. Understanding these properties is essential for grasping the complexity and diversity of matter.
Elements are characterized by their atomic number, which represents the number of protons in their nucleus. This fundamental property dictates an element's chemical identity and its position on the periodic table. Additionally, elements have specific atomic masses, isotopes, and electron configurations that influence their chemical behavior.
Metals, such as iron and copper, are known for their conductivity, malleability, and luster. These properties make them invaluable in construction, electronics, and manufacturing. Nonmetals, like carbon and sulfur, exhibit a wide range of properties, from gases to solids, and play crucial roles in biological and environmental processes.
Metalloids, including silicon and arsenic, possess intermediate properties, making them useful in semiconductors and other technological applications. Their ability to conduct electricity under certain conditions makes them vital in the electronics industry.
The periodic table also includes noble gases, known for their inertness and stability. Elements like helium and neon are used in lighting, cooling, and other applications due to their lack of reactivity. This stability contrasts with the reactivity of alkali metals and halogens, which readily form compounds with other elements.
Musical Interpretation of Elements
The musical interpretation of elements transforms the periodic table into a melody, capturing the essence of each element through rhythm and tune. This creative approach allows learners to engage with chemistry in a new and exciting way, making the subject more relatable and memorable.
Musicians and educators collaborate to assign musical notes and patterns to elements, often considering their properties and historical significance. For example, the lightness of hydrogen might be represented by a high, airy note, while the heaviness of lead could be conveyed through a deep, resonant tone.
This musical adaptation not only aids in memorization but also stimulates curiosity and creativity. By associating elements with music, learners are encouraged to explore the connections between different disciplines, fostering a more holistic understanding of science and art.
The "song of elements in the periodic table" serves as an educational tool that breaks down barriers between subjects, promoting interdisciplinary learning. It demonstrates that knowledge is interconnected, and understanding one field can enhance comprehension in another.
Overall, the musical interpretation of elements enriches the learning experience, making chemistry more engaging and inspiring for students of all ages.
Educational Benefits of the Song
The "song of elements in the periodic table" offers numerous educational benefits, making it a valuable resource for teachers and students alike. By incorporating music into chemistry education, this innovative approach enhances learning and retention in several ways.
Firstly, the song aids in memorization. The melody and rhythm provide a mnemonic device that helps students remember the names, symbols, and order of elements. This is particularly useful for younger learners who may struggle with traditional memorization methods.
Secondly, the song promotes active engagement. Music captures attention and stimulates interest, encouraging students to participate in the learning process. This active involvement leads to a deeper understanding of the material and a more positive attitude toward chemistry.
The song also caters to different learning styles. Auditory learners, in particular, benefit from hearing the elements set to music, while kinesthetic learners may engage with the song through movement or dance. This flexibility makes the song a versatile tool in diverse educational settings.
Moreover, the "song of elements in the periodic table" fosters creativity and interdisciplinary thinking. By integrating music and science, students are encouraged to think outside the box and explore connections between subjects. This approach aligns with modern educational practices that emphasize critical thinking and problem-solving skills.
Incorporating the song into the classroom can also enhance social learning. Group activities, such as singing or performing the song together, promote collaboration and communication among students. This social interaction can improve teamwork skills and create a supportive learning environment.
Notable Elements in the Song
The "song of elements in the periodic table" highlights several notable elements, each with its own significance and story. By focusing on these elements, learners gain a deeper appreciation for the diverse applications and historical importance of the periodic table.
Hydrogen, the first element, is often featured prominently in the song. As the most abundant element in the universe, hydrogen plays a crucial role in the formation of stars and the production of energy through nuclear fusion. Its simplicity and ubiquity make it a fundamental component of the natural world.
Carbon, another key element, is the backbone of life on Earth. Its ability to form complex molecules and compounds underpins organic chemistry and the study of living organisms. The song may highlight carbon's versatility and importance in biological processes.
Oxygen, essential for respiration and combustion, is another element that often takes center stage. Its vital role in supporting life and driving chemical reactions makes it an indispensable part of the periodic table's symphony.
Transition metals, such as iron and copper, are also noteworthy in the song. These elements are prized for their conductivity, strength, and versatility in industrial applications. Their inclusion in the song emphasizes their significance in shaping modern technology and infrastructure.
Lastly, the noble gases, known for their stability and inertness, provide a unique perspective in the song. Elements like helium and neon are used in diverse applications, from lighting to cooling, highlighting the practical uses of these unreactive elements.
By emphasizing these notable elements, the "song of elements in the periodic table" provides a comprehensive overview of the periodic table's diversity and relevance.
Creative Approaches to Learning Elements
The "song of elements in the periodic table" is just one example of the many creative approaches available for learning about elements. Educators and students can explore a variety of methods to make chemistry engaging and accessible.
One popular approach is the use of visual aids, such as color-coded charts and diagrams, to help students visualize the periodic table's structure. These tools can highlight trends and patterns, making it easier for learners to understand the relationships between elements.
Interactive activities, such as games and puzzles, are also effective in reinforcing knowledge. By challenging students to match elements with their properties or solve chemistry-related problems, these activities promote critical thinking and problem-solving skills.
Hands-on experiments provide another avenue for exploring elements. By conducting simple experiments, students can observe chemical reactions firsthand and deepen their understanding of elemental properties. This experiential learning approach fosters curiosity and encourages scientific inquiry.
Storytelling and historical context can also enhance learning. By sharing the stories behind elements' discoveries and their impact on society, educators can make chemistry more relatable and engaging for students.
Finally, technology offers innovative ways to learn about elements. Online resources, simulations, and virtual labs provide interactive experiences that allow students to explore the periodic table in new and exciting ways.
By combining these creative approaches, educators can create a dynamic and inclusive learning environment that caters to diverse learning styles and interests.
Impact on Science Education
The integration of the "song of elements in the periodic table" into science education has a profound impact on how students perceive and engage with chemistry. This innovative approach challenges traditional teaching methods, offering a fresh perspective on learning.
By incorporating music into the curriculum, educators can create a more inclusive and engaging learning environment. The song appeals to auditory learners and can make complex concepts more accessible to students who may struggle with conventional teaching methods.
The song also promotes active learning, encouraging students to participate and engage with the material. This active involvement leads to a deeper understanding of the subject and fosters a positive attitude toward science education.
Furthermore, the "song of elements in the periodic table" supports interdisciplinary learning. By integrating music and chemistry, students are encouraged to think creatively and explore connections between different fields of knowledge. This approach aligns with modern educational practices that emphasize critical thinking and problem-solving skills.
The song's impact extends beyond the classroom, inspiring a lifelong love for learning and scientific exploration. By making chemistry more relatable and enjoyable, the "song of elements in the periodic table" has the potential to inspire future generations of scientists and innovators.
Future of Music and Science Integration
The fusion of music and science, as exemplified by the "song of elements in the periodic table," represents a growing trend in education that emphasizes creativity and interdisciplinary learning. As technology continues to advance, the potential for integrating music and science in innovative ways is vast.
One promising area for future development is the use of digital tools and platforms to create interactive musical experiences. Virtual reality (VR) and augmented reality (AR) technologies can provide immersive environments where students can explore the periodic table through music, enhancing engagement and understanding.
Collaboration between musicians, educators, and scientists can lead to the creation of new educational resources that blend music with scientific content. These collaborations can result in a diverse range of materials, from songs and performances to multimedia presentations and interactive apps.
The integration of artificial intelligence (AI) in education offers additional opportunities for personalized learning experiences. AI-powered platforms can adapt musical content to individual learning styles, providing customized support and resources for students.
Furthermore, the rise of online learning and open educational resources (OER) allows for greater accessibility and dissemination of music-science integration materials. Educators and students worldwide can access and contribute to a growing repository of resources that enhance science education through music.
As the future of music and science integration unfolds, it holds the potential to transform education by making it more engaging, inclusive, and interdisciplinary. By embracing this trend, educators can inspire the next generation of learners to explore the wonders of science through the universal language of music.
Challenges in Creating the Song
The creation of the "song of elements in the periodic table" presents several challenges that require careful consideration and collaboration between musicians, educators, and scientists. These challenges must be addressed to ensure the song's effectiveness as an educational tool.
One of the primary challenges is accurately representing the elements and their properties through music. Assigning appropriate musical notes and patterns to each element requires a deep understanding of both chemistry and music theory. This process can be complex and time-consuming, as it involves balancing scientific accuracy with musical creativity.
Another challenge is ensuring that the song is engaging and accessible to a wide range of learners. The melody and rhythm must be catchy and memorable while also conveying essential information about the elements. Achieving this balance requires a thoughtful approach to songwriting and composition.
The song must also be adaptable to different educational settings and learning styles. Creating versions that cater to various age groups, language preferences, and cultural contexts can enhance the song's reach and impact. This adaptability requires collaboration and input from diverse stakeholders in education and music.
Additionally, the song's integration into the curriculum may face logistical challenges, such as aligning with educational standards and obtaining support from schools and educators. Overcoming these hurdles requires advocacy and demonstration of the song's educational benefits.
Despite these challenges, the "song of elements in the periodic table" has the potential to be a valuable educational resource that enriches science education and inspires a love for learning. By addressing these challenges, creators can ensure the song's success and relevance in diverse educational contexts.
Success Stories of Using the Song
The implementation of the "song of elements in the periodic table" in educational settings has led to numerous success stories, showcasing its potential to enhance learning and engagement in chemistry education.
In one case, a middle school science teacher incorporated the song into her classroom routine, using it as a warm-up activity before chemistry lessons. Students reported increased confidence in recalling element names and symbols, and their test scores improved significantly. The song's catchy melody and engaging lyrics made it a favorite among students, fostering a positive attitude toward chemistry.
Another success story comes from a high school chemistry club that used the song to prepare for a regional science competition. By practicing the song together, club members strengthened their understanding of the periodic table and improved their teamwork skills. The group went on to win first place in the competition, attributing their success to the song's role in their preparation.
In a college-level chemistry course, the song was used as an alternative assessment tool. Students were tasked with creating their own musical interpretations of elements, encouraging creativity and critical thinking. This project resulted in high levels of student engagement and a deeper understanding of the elements' properties and relationships.
These success stories highlight the song's versatility and effectiveness as an educational resource. By making chemistry more accessible and enjoyable, the "song of elements in the periodic table" has the potential to inspire a lifelong love for science and learning.
Criticism and Limitations
While the "song of elements in the periodic table" offers numerous educational benefits, it is not without its criticisms and limitations. Addressing these concerns is essential for maximizing the song's effectiveness as a learning tool.
One criticism is that the song may oversimplify complex concepts, leading to a superficial understanding of chemistry. While the song helps with memorization, it may not provide the depth of knowledge required for advanced study. Educators must supplement the song with additional resources and explanations to ensure comprehensive learning.
Another limitation is that the song may not appeal to all learners. While auditory learners may benefit from the musical format, others may prefer visual or hands-on approaches. Educators should incorporate a variety of teaching methods to accommodate diverse learning styles and preferences.
Additionally, the song's accuracy and relevance may be challenged as new elements are discovered and the periodic table evolves. Regular updates and revisions are necessary to ensure the song remains current and scientifically accurate.
The song's integration into the curriculum may also face resistance from educators who are unfamiliar with or skeptical of unconventional teaching methods. Providing evidence of the song's educational benefits and offering training and support can help overcome this barrier.
Despite these criticisms and limitations, the "song of elements in the periodic table" remains a valuable tool for enhancing chemistry education. By addressing these concerns and integrating the song with other teaching strategies, educators can create a dynamic and inclusive learning environment.
How to Use the Song in Classroom
Incorporating the "song of elements in the periodic table" into the classroom can enhance student engagement and understanding of chemistry. Here are some practical tips for using the song as an educational tool:
- Introduce the Song Early: Play the song at the beginning of a chemistry unit to familiarize students with the elements and spark interest in the subject.
- Use as a Memorization Aid: Encourage students to listen to the song regularly to help memorize element names, symbols, and positions on the periodic table.
- Incorporate into Lessons: Integrate the song into chemistry lessons by highlighting specific elements and their properties, using the song as a reference point.
- Facilitate Group Activities: Organize group activities where students perform or create their own versions of the song, promoting collaboration and creativity.
- Assess Understanding: Use the song as an alternative assessment tool, challenging students to explain the connections between elements and their musical representations.
- Supplement with Additional Resources: Provide supplementary materials, such as visual aids and hands-on experiments, to deepen students' understanding of the periodic table.
By incorporating the "song of elements in the periodic table" into the classroom, educators can create a dynamic and engaging learning environment that fosters a love for chemistry and science.
FAQs
1. What is the "song of elements in the periodic table"?
The "song of elements in the periodic table" is a musical composition that sets the elements of the periodic table to a melody, making it easier for students to memorize and understand the properties and order of elements.
2. How does the song benefit students in learning chemistry?
The song aids in memorization, promotes active engagement, caters to different learning styles, and fosters creativity and interdisciplinary thinking, enhancing students' overall understanding and appreciation of chemistry.
3. Can the song be used in different educational settings?
Yes, the song is adaptable to various educational settings, including classrooms, science clubs, and online learning platforms, making it a versatile tool for diverse learning environments.
4. Are there any criticisms of the song?
Critics argue that the song may oversimplify complex concepts and may not appeal to all learners. However, these limitations can be addressed by supplementing the song with additional resources and teaching methods.
5. How can educators integrate the song into their curriculum?
Educators can introduce the song early, use it as a memorization aid, incorporate it into lessons, facilitate group activities, assess understanding, and supplement it with additional resources to enhance chemistry education.
6. What is the future of music and science integration in education?
The future of music and science integration is promising, with opportunities for using digital tools, VR/AR technologies, and AI-powered platforms to create interactive and personalized learning experiences that blend music with scientific content.
Conclusion
The "song of elements in the periodic table" offers a unique and innovative approach to learning chemistry, transforming the study of elements into an engaging and memorable experience. By setting the periodic table to music, this educational tool enhances memorization, promotes active engagement, and fosters creativity and interdisciplinary thinking.
While the song has its criticisms and limitations, its potential to enhance science education is undeniable. By addressing these concerns and integrating the song with other teaching strategies, educators can create a dynamic and inclusive learning environment that inspires a lifelong love for science.
As the future of music and science integration continues to evolve, the "song of elements in the periodic table" serves as a testament to the power of creativity and innovation in education. By embracing this trend, we can inspire the next generation of learners to explore the wonders of science through the universal language of music.
For more information on the periodic table and its educational applications, visit the Royal Society of Chemistry's website at rsc.org/periodic-table.